The term which best describes the crystalline substance that results when a large number of metal atoms transfer electrons to a large number of non-metal atoms is. Titration of 5000g of vinegar with 0100 NaOH requires 330 ml to reach.
The Ph Scale Biology For Non Majors I
Sodium Na and chlorine Cl combine to form sodium chloride NaCl which is commonly known as.

. Chemistry - a substance that has the opposite effect or chemical behavior of an acid. The backbone of all protien molecules is the. Considering the cost of alkaline water some of the simple but proven methods of reducing cancer risk such as eating healthy diet and exercising may be more worthwhile.
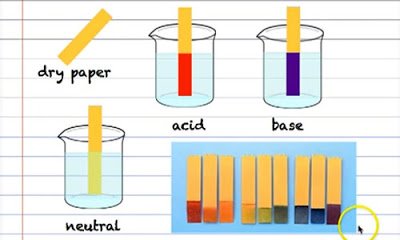
A d-transition metals b representative elements c metalloids d alkaline earth metals e halogens 3. A Word From Verywell. Indicators are used to determine whether a solution is acidic or alkaline.
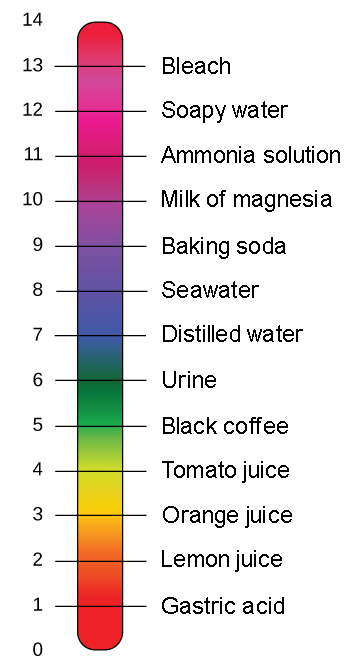
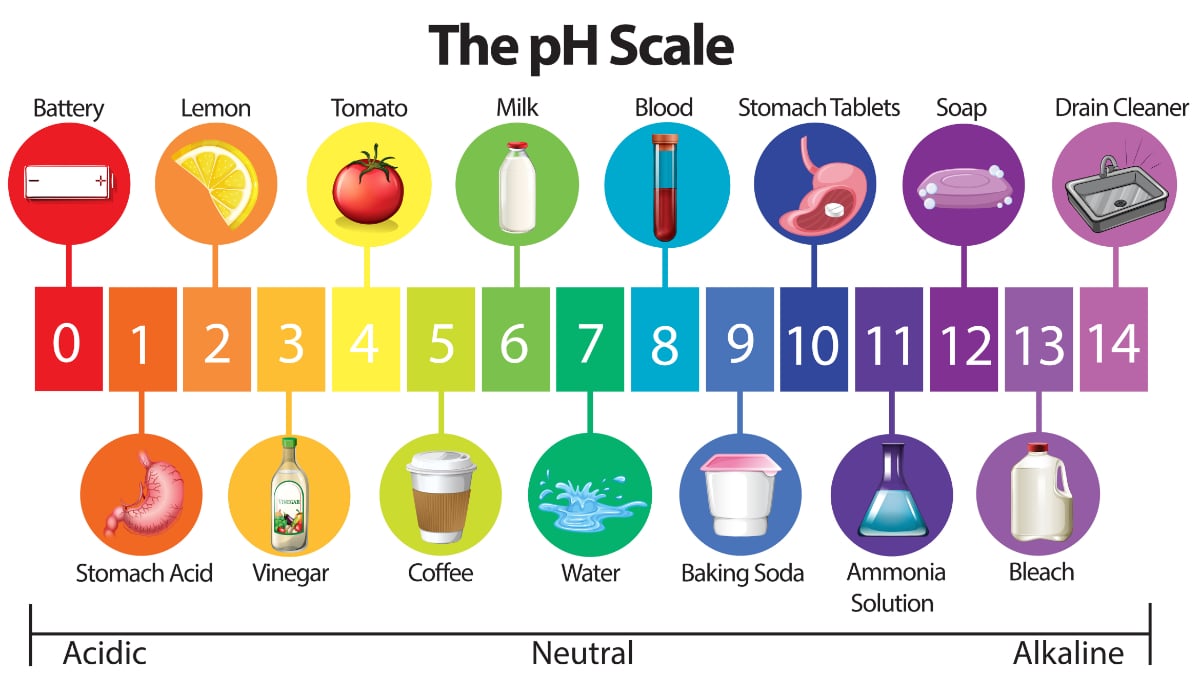
The modern chemistry sense is from 1813. We can find out how acidic or basic a substance is by using the pH scale. A high pH and a high pOH.
Which describes an alkaline solution. Substances of an acidic or alkaline nature dissolve in water and or contain. There are many substances that are alkaline such as soap toothpaste dock leaves and detergents.
A low pH and a high pOH. Any substance that has a pH higher than 7. When added to water both can form solutions having higher pH values pH.
Wood and plant materials. A low pH and a low pOH. Hydrogen is found twice on both sides of the equationB.
A high pH and a low pOH. Is it true that A substance with equal numbers of H ions and OH- ions is an alkaline solution. A soulution is acidic if it has more negative hydroxide ions.
Most solutions are either acidic or basic also called alkaline - a substance that is neither acidic nor a basic is neutralAcids and bases are very important chemically and are found almost everywhere. Here are a total of. The greater the pH number is the more basic the substance is.
Which statement best describes renewable resources. Neutralisation is the reaction between an acid and a base. Acids react with metals bases and carbonates to produce salts.
Our new pH values are then solution A 125 solution B 70 and solution C 05. The main difference between alkali and alkaline is that alkali metals have one valence electron whereas alkaline earth. A solution containing HCl would likely have.
Get the answers you need now. Alkaline batteries have the advantage of putting out constant voltage until very nearly the end of their life. A Li b Na c Rb d F e I 4.
Search for an answer or ask Weegy. Acids react with metals bases and carbonates to produce salts. Both have basic properties.
Alkaline as a adjective means Having a pH more than 7. Just as an example a substance with a. An alkaline solution is formed when an alkali is dissolved in water.
In order to convert from pOH to pH we can use the equation pH 14 - pOH. The best way to separate salt from water is with the use of. Alkali metals are the elements in the group 1 of the periodic table.
A vinegar contains acetic acid CH3COOH. Select the term best describing the series of elements. Which of the following best describes a strong electrolyte.
The term pH is an abbreviation for power of Hydrogen which is a measure of how acidic or basic a chemical solution is. Small E none of the above. Which element has the largest atomic radius.
This is called neutral and is right in the middle of the scale. The same number of atoms of each substance is present on both sides of the equationD. The probability of finding an electron at any particular point in an atom.
Any substance which is to the left of this point on the scale is considered acidic. PH less than 7 pH equal to. Neutralisation is the reaction between an acid and a base.
Later extended to similar substances natural or manufactured. Adhesion--the process of water being attracted or adhering to other substances. All substances whose pH falls to the right of 7 on the scale are basic.
Overall based on studies to date alkaline water appears to have little effect on the development or progression of cancer. Alkaline solutions are bitter tasting and feel slippery when touched. How long will an alkaline battery rated at 1 A h and 158 V keep a 1 W flashlight bulb burning.
Which of the following terms accurately describes the energy associated with the. Mn Fe Co Ni Cu. Which describes an alkaline solution.
Pure distilled water has a pH of 7. The log of the hydroxide ion concentration. Chemistry Having the reactions of an alkali.
Also metal hydroxides are alkaline NaOH KOH Ca OH2 etc. An element belonging to the alkaline earth family would be expected to have a _____ ionization energy and a _____ electron affinity. Group of answer choices A substance that is completely ionized in solution.
The term alkaline is derived from the metal elements of group 1 and group 2 in the periodic table of elements. Scientists can identify and element by looking at the structure of a single. Alkalinity--the capacity of water for neutralizing an acid solution.
Alkaline--sometimes water or soils contain an amount of alkali strongly basic substances sufficient to raise the pH value above 70 and be harmful to the growth of crops. Alkali refers to any basic hydroxide or a salt of alkali metals or alkaline earth metals. Saltwater vinegar bronze air and beach sand are in which category.
All of the following are true regarding PH EXCEPT. Alkaline earth metals are the elements in the group 2. Based on the definition of logarithms what is the difference in hydrogen ion concentration between a substance with a pH of 2 and a substance with a.
Indicators are used to determine whether a solution is acidic or alkaline. A substance that does not ionize in water and consequently gives a nonconducting solution.
Igcse Acids Bases Salts Quiz Quiz Quizizz
Water Quality 101 What Is Ph In Water Testing

Difference Between Ebullioscopic Constant And Cryoscopic Constant 2 Chemical Renaissance And Reformation Electrons
0 Comments